APEC High-Level Policy Dialogue on Agricultural Biotechnology
Policy Approaches Document for Regulatory Cooperation on Agricultural Biotechnology
WORKING DRAFT
Last Updated:
October 15, 2024
I. Introduction
The announcement of the first successful transformations of plants with DNA from bacteria in 19831 kicked off a series of scientific and agricultural breakthroughs that would see the wide scale introduction of genetically engineered (i.e., transgenic or “genetically modified”) plants for use in agriculture. Within a decade, the first products were entering into regulation, capturing the public consciousness, and kicking off discussions about the role of science and technology in society, and food production in particular, that had previously been relatively uncontentious. Within 20 years, genetically engineered crops would occupy significant percentages of the total global acreage of corn, soy, cotton, and canola (oilseed rape) production. By 2011, acreage in developing economies exceeded production acreage in industrial economies.2 After 40 years, it is now almost expected that, when these technologies are available to farmers, these crops will be planted on 90% or more of production acreage. It is among the fastest adoptions of a technology for food production in human history.
These scientific achievements were accompanied by critical discussions about the nature of new technologies and the means to ensure that their benefits could be realized safely for humans and animals. Almost as soon as the first successful plant transformations were announced, regulators and policy makers began to engage on whether and how to properly ensure safety. The earliest policy frameworks were taking shape by 1986,3 and these were accompanied from the onset by international cooperation. In support of nascent biotechnology regulatory policies, the Organisation for Economic Cooperation and Development (OECD) published “Recombinant DNA Safety Considerations” in 1986. Known as the “Blue Book” for its distinctive blue cover, the text introduced to a broader audience a variety of the core concepts still used in regulatory risk/safety assessment for biotechnology. It also illustrates that the development and deployment of agricultural biotechnology has been accompanied by regulatory cooperation since the very beginning.
Throughout the 1990s, as experience in development, regulation, and release of biotech crops increased, international regulatory cooperation expanded. In 1997, language around risk assessment for biotechnology was adopted in the International Plant Protection Convention (IPPC). Concepts and mechanisms first described in the OECD Blue Book were incorporated into Annex III on risk assessment of the Cartagena Protocol on Biosafety (CPB), an international agreement adopted in 2003 for transboundary movement of organisms derived from “modern biotechnology” that may have impacts on biodiversity. One goal of the CPB is to address the gap between Parties with well developed regulatory processes and Parties who had not yet developed a legal or regulatory framework for decision-making. As such, the CPB contains a mechanism for decision making, accompanied by risk assessment, that can be used by Parties in the absence of a domestic framework. Although regulatory cooperation efforts at the international level are not always well coordinated, the CPB shares a definition of “modern biotechnology” with the Codex Alimentarius (Codex), which also drafted and published guidelines for food safety assessment of foods derived from recombinant DNA plants, animals, and microbes. The Codex guidelines, initially adopted in 2003 and updated since, represent the culmination and convergence of cooperative efforts around food safety standard setting under the World Trade Organization (WTO) and cooperation in the regulation of agricultural biotechnology. They also provide a solid foundation for enabling future cooperative efforts by establishing a shared standard for considerations of food safety assessment for biotechnology.
The APEC High Level Policy Dialogue on Agricultural Biotechnology (HLPDAB)
The HLPDAB represents a forum for economic and regulatory cooperation among APEC member economies. Member economies participate in order to exchange information, improve clarity and transparency on regulatory polices and challenges, and work towards reducing the burden on member economies when dealing with agricultural biotechnology. Within this forum, the idea of advancing regulatory cooperation is not new. The forum has long been interested in improving clarity around biotechnology regulations and policies within APEC economies, and a Baseline Study: Regulations of Products Derived from Innovative Agricultural Technologies and Identification of Ways to Promote Greater Efficiencies and Alignment (Baseline Study) was first published in 2006, updated in 2016 and again in 2018, and published in 2019.4 The Baseline Study and its updates recognize key findings that remain true in the latest consultations under the HLPDAB. The capacity and experience for regulating agricultural biotechnology varies between member economies. For those that have an established regulatory structure, however, these tend to share commonalities–particularly around the technical requirements for risk/safety assessment, as well as for compliance with the Codex guidelines and an adherence to international standards.
Findings of the 2019 Update to the APEC Baseline Study
- Finding 1. Almost all APEC Members indicate that they adhere to international standards for the assessment of GM food products. However, there is variability in the decision-making process for GM food products. This is largely in the predictability of assessment, transparency of the process, certainty of an approval, and the historical experience of economies in undertaking assessments and issuing approvals.
- Finding 2. Regulatory cooperation and harmonization do not compromise domestic autonomy but do require political will to implement. GM products are still regarded as controversial by many actors, and as such, there is often a lack of desire to change the status quo and maintain a precautionary approach, despite the overwhelming evidence that supports the more than 20 years of benefits.
- Finding 3. In implementing a precautionary approach, regulatory agencies often seek more data from developers (as is a requirement under the SPS Agreement). However, for GM food products, there is a lack of evidence that more data, at higher cost, provides a higher level of safety or certainty.
- Finding 4. The familiarity of GM food products, particularly with certain trait and species combinations, should enable streamlined approaches for regulatory assessment. The Health Canada (HC)/Food Standards Australia New Zealand (FSANZ) GM food safety assessment sharing model highlights the benefits of regulatory cooperation and serves to highlight the opportunities and benefits for those economies that are prepared to embark on a cooperation program.
- Finding 5. Four key tenets to regulatory cooperation have been identified: trust, benefit, fairness, and control.
- Finding 6. The regulatory systems of APEC Members were compared to identify economies suitable for regulatory cooperation activities. Differences between regulatory systems were classified into five categories.
- Finding 7. Two economies (Malaysia and Singapore) stood out with the potential to explore a program of work similar to HC and FSANZ. This approach provides a model for food safety assessment sharing that could also be adopted by other APEC Members. However, it is noted that preconditions to this approach include the regulatory coherence of each economy, a willingness and commitment to cooperate from senior leaders within each regulatory agency, a program of work that builds trust, and an allocation of time and resources to ensure program momentum is maintained.
- Finding 8. Many APEC Members would benefit from further regulatory coherence activities that lay a foundation for future regulatory cooperation and, ultimately, regional harmonization.
Since the latest update to the Baseline Study, the HLPDAB has focused activities, including intercessional webinars and workshops affiliated with the annual convening of the HLPDAB, on laying the foundation for further collaboration. This includes building a relationship of trust among participating member economies, raising awareness of the potential benefits of increased cooperation, continuing to share information and developments from within member economies, and improving both awareness and understanding of emerging technologies such as genome editing.
Efforts to develop these activities into a practical approach to regulatory cooperation took shape following the meeting of the HLPDAB in 2022. As a result, the outline for a “Policy Approaches Document” was presented at the HLPDAB meeting in 2023 and elaborated through a series of consultations into this document and the accompanying Policy Approaches Web Portal presented to the HLPDAB during SOM3 in Trujillo, Peru.
The following sections, informed by a series of specific consultations, as well as the ongoing activities of the HLPDAB and member economies, include a discussion of the potential benefits for greater cooperation and alignment between APEC economies, an elaboration of some of the mechanisms for regulatory cooperation that might be explored, and a database of examples illustrating practical approaches to regulatory cooperation that may be of interest to APEC member economies. These examples, together with the text of this Policy Approaches Document, will be captured on the Policy Approaches Web Portal and are intended to be a “living” resource for HLPDAB members. Case studies will be added to the database of examples of regulatory cooperation and updates to the text can be made as needed.
II. Potential Benefits of Greater Cooperation and Alignment of Agricultural Biotechnology Policies and Regulations in APEC Economies
Regulatory cooperation and alignment provide an opportunity for APEC member economies to realize a variety of potential benefits, based both on general principles and benefits of cooperation, as well as on the results of specific benefits of cooperation related to agricultural biotechnology. These benefits can be immediate, short, and long term, and may accrue substantially over time. It is important to consider the benefits and the lasting potential for benefit over time because regulatory cooperation also comes with a cost. Typically, these costs are in the form of making changes or adaptations to existing regulatory frameworks and may include both a human resource cost (for example staff time for development and finalization of regulatory changes), as well as a political cost (the time and attention of policy makers and officials necessary to make changes to regulatory mechanisms).
While the benefits of regulatory cooperation are generally well recognized, efforts to achieve practical harmonization are often stymied by the simple truth that the costs (in the form of effort) for regulatory cooperation are immediate, while the benefits are more often accrued over time. For example, the OECD reports that the financial benefits from regulatory cooperation around chemicals regulation result in savings of over €317 Million/year based on quantifiable savings to the chemicals industry. Comparatively, the budget for OECD’s harmonization work, estimated at under €9 Million/year, is clearly well justified. However, the budget for these harmonization efforts is also an easily identifiable “cost” to the governments funding OECD. As a result, despite the obvious success of these programs, investments in OECD are frequently the subject of discussion for budget cuts and efforts to reduce spending. Importantly, none of the benefits included in OECD’s analysis incorporate estimates of the benefits accrued to other stakeholders. For example, the availability of safe chemical alternatives and the distribution of uniform safety information have real benefits for the health and economic well-being of farmers and consumers, but these can be difficult to quantify.
This reality has been reflected in multiple rounds of engagement at the APEC HLPDAB, where participants frequently identified “political will” as a barrier to practical implementation of regulatory cooperation. It is therefore useful to explore the expected breadth and depth of benefits over time in order to allow APEC economies to make rational decisions about the types and extent of regulatory cooperation efforts they might engage in.
The benefits of greater cooperation and alignment of agricultural biotechnology policies and regulations can be categorized in any number of ways. Here we will briefly explore the generally recognized benefits of regulatory cooperation, which primarily accrue to governments and regulators, as well as to developers who are applicants to regulatory systems. Then, we will take a brief look at how regulatory cooperation can provide additional and often unrecognized benefits to governments (including regulators and policymakers), developers, consumers, traders and other participants in the supply chain, and farmers (and other agricultural producers).
II. A. General Benefits of Regulatory Cooperation
Regulatory cooperation provides generally recognized benefits essentially through improvements in efficiency. This means savings in cost and time. Feedback collected through dialogue at the APEC HLPDAB workshops and the regulatory consultations that have informed this publication identified “efficiency” and “cost savings” as the two most frequently identified benefits by APEC economy participants. The theory behind this is quite simple, if economies are operating in a harmonized way, then the duplication of effort by both applicants and regulators is reduced.
It is important to note that this is true even for relatively passive methods of regulatory cooperation. For example, alignment of data requirements can allow applicants to assemble a single data package that can be used in multiple jurisdictions. Similarly, regulators benefit from aligned data requirements because they are likely to see uniformly prepared applications organized around those aligned requirements, reducing the time needed to review and likely also time required to explain requirements to applicants and ask for revisions or corrections to deficiencies and other administrative measures related to helping applicants understand data requirements. Applicants may additionally save time and money by having to conduct fewer tests if requirements are aligned.
In addition to improving efficiency, a significant benefit of regulatory cooperation comes from reduced inefficiencies and vulnerabilities associated with trade disruptions that can cause economic harm. Disparate and asynchronous approvals can complicate trade and reduce efficiency of commodity production by requiring efforts to segregate or restrict the movement of agricultural products to jurisdictions where authorizations are in place. This works against the efficiencies inherent in commodity trade that provide economic advantage to producers, traders, consumers, and economies as a whole. Regulatory cooperation makes synchronous approvals more likely, reduces the likelihood of trade disruption, and can decrease the severity and duration of trade disruptions by allowing for swift review and decision-making for products and commodities that have been subject to review in another economy.
Benefits to Regulators
As discussed above, the benefit most often considered for regulators is a savings of time and effort. Depending on the type of regulatory cooperation and the administration of the regulations, this can include reducing the time spent analyzing an individual application or even removing the need to review an application that has already been reviewed somewhere else. More commonly, regulatory cooperation leads to more standardized applications that have predictable data presented in a predictable way, which facilitates review. This does not provide a complete picture, however, in part because time savings must be balanced against time dedicated to regulatory cooperation. While most cooperative efforts take less effort than reviewing dossiers, there is still a time commitment necessary to achieve and often maintain functional regulatory cooperation. As evidenced in the OECD experience with chemical regulations, the cost of those efforts are small compared to the accrued savings, but it is still important for economies hoping to benefit from regulatory cooperation to understand that some amount of effort will be required to develop the cooperation and maintain it.
There are also intangible benefits that regulators accrue through cooperative efforts. These are benefits that may be difficult to quantify but are no less real. Primary among these is increasing the amount of knowledge, experience, and expertise regulators have access to through engagement with regulators from other economies and the value of enhanced understanding that emerges from technical discussions and negotiations around the regulatory cooperation. For example, the OECD publication “Considerations for Collaborative Work on Safety Assessments of Foods and Feeds Derived from rDNA Plants” (OECD, 2023) identifies “mutual capacity building and learning,” as a benefit, highlighting the “opportunity for sharing knowledge between agencies and enhancing professional development of staff using actual submissions,” as well as the opportunity for regulators with less experience to learn from more experienced colleagues. In the example of regulatory cooperation involving Health Canada and FSANZ provided in the same document, regulators from those economies highlighted that, despite a high level of existing capacity for safety assessment, their collaboration has provided an opportunity for continued learning. While these benefits are difficult to value monetarily, they can result in increased confidence in regulators and regulatory decisions.
Benefits to Developers
Benefits to developers are often the easiest to quantify because many are direct, in the form of reduced costs for duplicative testing and reduced time and resources spent to assemble applications. However, developers also benefit from regulatory cooperation through a reduction in decision times and improved predictability of regulatory decision-making when regulatory cooperation provides for more harmonized processes. These benefits also make investments in research and development less risky and encourage innovation and the development of new products, which can, in turn, provide benefits to farmers and consumers.
We often think about the benefits to developers in the context of multinational companies because these are the developers typically submitting multiple applications across many regions and economies. Benefits of regulatory cooperation can also be realized by small developers, startups, and public sector researchers who have developed agricultural biotechnology products that they plan to release only in their local economy or a small subset of economies that have a shared need. Most directly, the improved regulatory clarity and predictability that results from regulatory cooperation can reduce uncertainty and speed up approvals for their applications. However, regulatory cooperation also helps by expanding the pool of knowledge and applications that can be referenced and that may be relevant to their own application.
For example, imagine an economy that has only issued a few decisions related to agricultural biotechnology products. It may be difficult for new or novice developers hoping to release a product in that economy to understand how to prepare and submit an application. If regulatory cooperation efforts have aligned elements of the technical and regulatory framework with other economies, then the local developer can also reference applications that have been submitted to those other economies with some confidence that their submission will be subject to a similar review.
Benefits to Agricultural Traders
In the modern context, agriculture is intertwined with an elaborate global trade network that works to move agricultural products from where they are grown to where they are needed. This supply chain involves local and international organizations, government and private tenderers, and procurement, and can be affected by import/export policies, sanitary and phytosanitary regulations, and other financial risks associated with commodity and agricultural markets. The value of agricultural trade is growing, rising from around $300 Billion USD in 2000 to more than $1.6 Trillion in 2022.5 It can also be heavily impacted by regulations for agricultural biotechnology. Regulatory cooperation can reduce the financial risks and uncertainties associated with asynchronous regulatory approvals, as well as reduce the costs associated with product segregation.
In addition to the immediate economic advantages provided by regulatory cooperation, predictable and harmonized approaches to agricultural biotechnology can also improve the ability of traders to respond to other disruptions and shocks. If production is disrupted in one economy, then it is much easier to find alternative sources of supply if economies have implemented measures to align regulatory requirements through regulatory cooperation. This also allows producers to more predictably respond to global market demand.
Benefits to Farmers and Agricultural Producers
Regulatory cooperation leads to benefits for farmers and other agricultural producers by reducing disruptions in the supply of seed and feed products that they depend on, as well as cost reductions that can be passed on through the reduction in the burden on developers and trade organizations. However, there are also more specific benefits to regulatory cooperation on agricultural biotechnology that can be seen by looking at the experiences in economies where farmers have access to these technologies. A meta-analysis of published reports by Areal et al. demonstrated that the products of agricultural biotechnology produced meaningful economic gains when compared to conventional products and that these gains were most evident in developing economies (Areal et al., 2013). With the understanding that products are beneficial, the opportunity to enhance the speed and predictability of regulatory processes through regulatory cooperation will then allow broader and faster access to these benefits from farmers and producers.
Benefits to Consumers
Ultimately, economic benefits of regulatory cooperation for developers, agricultural producers, and traders will also translate into economic benefits for consumers. This is typically seen in reduced prices, but consumers can also benefit from increased availability and diversity of agricultural products and the resilience of markets to price and production shocks. When regulatory cooperation encourages investment in research and development, consumers may also benefit from the availability of new products that match their needs or tastes, as well as the improved environmental and health impacts. For example, the use of agricultural biotechnology in North America has been associated with increased use of low and no-till agricultural systems that reduce erosion and agricultural run-off. This decreases the environmental impacts of nitrogen and fertilizer pollution as the water runs into lakes, rivers ,and ponds and can provide public benefits.
II. B. Relevance to APEC Economies and the HLPDAB
Regulatory cooperation is an investment. Regulatory agencies and economies invest time and effort into cooperation in order to see a return on that investment in the form of future savings in time and effort, as well as the intangible benefits described above. In order to help decision-makers and officials make informed decisions about how to allocate time and resources to cooperative efforts, it is necessary to provide them with evidence that those benefits will be realized and that they represent a good return on the invested effort.
The good news for APEC and the HLPDAB is that all of the available evidence suggests that the return on investment for regulatory cooperation is very high. The OECD’s analysis of annual savings from cooperative efforts around chemical regulation show a 30-fold annual return in the form of cost savings that can be reasonably accounted. This does not include any attempt to capture the value of more intangible benefits and those that accrue more indirectly to stakeholder groups within the OECD economies.
Feedback collected during the preparation of this document and during workshops conducted over the last two years indicates that there is appreciation among APEC member economies for the benefits that can be achieved as a result of regulatory cooperation. With that consensus, the task becomes determining which mechanisms and levels of effort are most appropriate for APEC and individual economies to engage in.
III. Approaches to Greater Cooperation and Alignment of Agricultural Biotechnology Policies and Regulations in APEC Economies: Potential Mechanisms of Regulatory Cooperation
There are many different approaches representing a range of usefulness and complexity that economies can use to enhance regulatory cooperation and alignment. This section explores some different options for economies to consider. Key approaches to regulatory cooperation are considered in three categories: 1) information sharing, 2) policy alignment, and 3) collaboration on risk/safety assessments. The details, merits, and inherent challenges of each of these different approaches are considered here. The relevance of each approach and perceived opportunities for APEC and the HLPDAB, based on input from the member economies through a series or webinar consultations in May/June 2024, are also described for each of these broader approaches.
III. A. Information Sharing
Information sharing is the most easily implemented and commonly employed approach to regulatory cooperation. It is simply the provision of relevant information by organizations or agencies between member economies, accomplished through general broadcasting of information or more targeted direct communication, either of which can be tailored to different situations to accomplish specific goals. Broadcasting may be general for “public communication” with no specific audience in mind, or it can be more limited to a select group. Information can also be shared directly by an individual or organization specifically to another individual or organization, not intended for sharing outside the targeted audience. In the case of direct communication, there is often the expectation of a response. Some different types of information sharing are described below. It should be noted that while information sharing can be an effective approach to regulatory cooperation, it will also occur implicitly as part of other more advanced approaches that are discussed in the sections on policy alignment and risk/safety assessment collaboration.
Agency Postings of Regulatory Information
Most regulatory authorities will make information publicly available for stakeholders through an agency website and/or by posting in national registers or other federal listings. Information shared will typically include policies, guidelines, and decisions, with more or less detail determined by the needs of the agency.
Biotechnology Regulatory-Related Databases
There are a number of databases that contain policies, risk/safety assessments, authorizations, and dossiers. For example:
- The Biosafety Clearing-House (BCH) of the Convention on Biological Diversity is an online platform for exchanging information on Living Modified Organisms (LMOs) and a key tool for implementation of the Cartagena Protocol on Biosafety.
- The Food and Agriculture Organization (FAO) has multiple databases and platforms related to biotechnology, including:
- FAO-BioDeC is a database that collects, organizes, and shares information on crop biotechnology products and techniques used or being developed in developing economies.
- FAO Biotechnology Forum is a platform that provides access to information and a neutral space for stakeholders to exchange views on agricultural biotechnologies in developing economies.
- FAO GM Foods Platform is a publicly accessible central database that contains information on recombinant-DNA plants authorized in accordance with the Codex Plant Guideline.
The International Service for Acquisition of Agri-biotech Applications (ISAAA) hosts the GM Crop Approvals database that includes all available information on biotech/GM crops that have been approved for food and feed use and/or cultivation globally.
The Organization for Economic Cooperation and Development (OECD) has several databases related to biotechnology, including:
- BioTrack Product Database allows stakeholders to share information about products from modern biotechnology and other products with novel traits that have been approved for commercial use in at least one member economy.
- Emerging Technology Indicators is a database that combines measures of the number of companies active in biotechnology, R&D expenditure, and inventions.
Data Sharing/Data Transportability
Member economies can establish standardized mechanisms for the sharing of regulatory data, and this can be a particularly effective form of regulatory cooperation. The concept of “data transportability” has gained interest among the regulatory and product developer communities in recent years. The concept is centered around the notion that data from well-designed regulatory studies following accepted methods for data collection, especially for comparison of GM plants to their conventional counterparts, submitted for a regulatory decision in one jurisdiction can be used for risk/safety assessment in another jurisdiction. Transportation of data for risk/safety assessments has been used effectively in multiple economies to increase regulatory efficiency and eliminate redundancy.
Information Sharing and Capacity Building
Regulatory coordination in the form of information sharing can also be accomplished by APEC member economies through arranged capacity building when member economies with more experience are willing to provide information on policies, processes, and decisions taken to economies with less experience through consultations, seminars, workshops, study tours, internships, and other methods.
Information Sharing - APEC Opportunities and Challenges
A webinar consultation on information sharing held on May 6, 2024 was attended by 16 participants from eight member economies: Australia, Canada, Indonesia, Japan, Peru, Philippines, USA, and Vietnam. A discussion with participants during this webinar provided some important insights regarding the value of and capacity for information sharing among member economies as a form of regulatory cooperation.
Below are key points from the consultation on information sharing as an approach to regulatory cooperation:
- Among APEC member economies, those that have made decisions on cultivation and/or food and feed use of GMOs do typically publish decisions in a national register and/or announce decisions on an agency website, and some share decisions with international bodies such as FAO and the CBD.
- Member economies are more likely to provide information to the CBD Biosafety Clearing House (BCH), and fewer to the FAO GM Foods Platform. Member economies are less likely to provide information to specific databases such as the ISAAA GM Crop Approval Database and the OECD Biotrack Database. While some APEC member economies do utilize some or all of these databases as information resources for decision-making, others are not familiar with any of these databases as a resource.
- Technical capacity, resources, and policy constraints (whether too strict or lacking) are potential barriers for information sharing and confidential information and public perception also raise significant concerns. APEC member economies would benefit from a dedicated online resource serving as a directory to the available databases and information resources, and/or a repository for existing regulations, guidelines, products approved, risk/safety assessments, and decision documents (ideally with the translation of documents into English).
- There are also opportunities for more direct communication between member economies, whether between two economies or between or within groups of economies with similar needs based on the status of their regulatory systems and level of experience. Member economies could benefit from sharing experiences of functional systems and processes for regulation of biotechnology, risk/safety assessments, and decision-making.
- Economies with experience handling certain issues, for example, the handling of confidential information identified as a potential barrier for information sharing in general, could work with other economies to develop a system for providing confidential information to each other. Because concerns about public perception are a potential barrier to sharing information with the public, member economies might benefit from best practices for communicating with the public and public engagement about regulation and regulatory decisions.
- Any number of case studies relevant for the HLPDAB and lessons learned, based on member economies’ experience around specific products with global impact, could serve as a catalyst for information sharing, ranging from best practices for assessing and managing risk/safety and regulatory approvals to best practices for public communication and managing public perception.
III. B. Policy Alignment
Alignment of policies and procedures is in itself an approach to regulatory cooperation, and it can be a necessary and important step toward achieving other forms of regulatory cooperation, in particular for efforts to harmonize risk/safety assessments among member economies, as discussed in the next section on collaboration on risk/safety assessments. Depending on the reason for cooperating, policy alignment can be pursued at different levels. High level policy alignment and lower-level technical policy alignment are discussed here.
High Level Policy Alignment
High level policy alignment may be the easiest to achieve. Most economies will have established a similar high-level policy for biotechnology, along the lines of “to realize the potential benefits of biotechnology while ensuring safety for humans and the environment, organisms derived using modern biotechnology are subject to risk/safety assessment prior to introduction to the market.” This is also consistent with the international obligations for economies that are party to the Cartagena Protocol on Biosafety under the Convention on Biotechnology. However, if economies will strive to achieve policy alignment at a lower level, such as technical policies or policies to streamline regulatory cooperation, it is essential to acknowledge alignment of higher-level policies first. The existence of the APEC High Level Policy Dialogue on Agricultural Biotechnology suggests there is already at least some degree of high-level policy alignment within APEC economies.
Technical Policy Alignment
At another level, technical policies are the “mechanisms” put in place to accomplish higher level policies. Technical policies may refer to administrative procedures or scientific technical procedures, such as those for risk/safety assessment. Administrative procedures include defined timelines for issuing decisions or defined steps and required committees for reviewing applications. Alignment of administrative procedures may not be necessary for regulatory cooperation although it will always be important to carefully consider similarities and acknowledge differences in administrative procedures before economies embark on efforts to align technical policies for risk/safety assessments.
Technical policies related to risk/safety assessment include methodologies or ways to conduct risk/safety assessments and scientific or technical requirements for generating information or evidence in support of an assessment. Most experienced regulatory systems will have established “tools” for risk/safety assessment that can be used as a basis for comparison and a catalyst for alignment among economies, such as guidance on the conduct of assessments, agreements on the types of information (data) needed to inform risk/safety assessments, criteria for data acceptance or guidelines for testing, or standardized templates for application or data submission. Alignment of risk/safety assessment policies may be the most impactful approach to regulatory cooperation but may also bring significant challenges, as discussed in more detail in the section on risk/safety assessment collaboration.
Alignment of Streamlined Policies
As biotechnology regulatory systems mature, experience makes it possible to streamline or modify policies in ways that employ faster or simpler methods to make the systems more efficient and effective. Policies may be streamlined for assessment and decision making, for example, in the cases of products that have been subject to earlier reviews or products that have other risk/safety mitigating properties. Simplified procedures represent another opportunity for policy alignment among member economies. In some cases, economies can consider alignment of a simplified procedure for certain cases even when there is not alignment of the more complex policies in each economy.
A good example of regulatory cooperation through the alignment of streamlined policies is the declaration on new breeding techniques signed in 2017 by the Ministers of Agriculture from Argentina, Brazil, Chile, Paraguay, and Uruguay, specifically with the goal to exclude certain gene edited products from strict regulation by adopting common streamlined policies (Turnbull et al., 2021). Among the economies in Latin America, Colombia, Honduras, and Ecuador have also drafted legislation for this purpose. In the case of Ecuador, the constitution does not allow cultivation of GM crops unless the president deems it to be in the interest of the nation, but the Ecuadorian government implemented a decree in 2019 excluding certain gene edited products from regulations associated with GMOs, similar to other economies.
International Policy Alignment
When member economies’ policies are aligned with established and accepted international policies, the alignment of policies between member economies becomes much more straightforward. Several international forums provide guidance and information that support consistent policies and assessments. Some of these are listed below:
- Codex Alimentarius
- OECD Working Party on Safety of Novel Foods and Feeds
- OECD Working Party on Harmonisation of Regulatory Oversight in Biotechnology
- International Plant Protection Convention
- World Organization for Animal Health
- Cartagena Protocol Annex III
APEC member economies may also work toward aligned policies and approaches through participation in international capacity building programs. For example, the Food and Agriculture Organization (FAO) conducts biosafety capacity building as part of its efforts to improve food security and agricultural practices globally. There are also a number of capacity building programs associated with the implementation of the Cartagena Protocol on Biosafety, including those supported by the United Nations Environmental Programme (UNEP) and Global Environment Fund (GEF). Capacity building programs exist to provide support for policy development, risk assessment training, development of biosafety guidelines, and some to foster regional cooperation specifically.
Policy Alignment - APEC Opportunities and Challenges
A webinar consultation on consistent policies and procedures held on May 29, 2024 was attended by 14 participants from eight member economies: Australia, Canada, Indonesia, Japan, Peru, Philippines, USA, and Vietnam. A discussion with participants during this webinar provided some important insights regarding the value of and capacity for alignment of policies and procedures among member economies as a form of regulatory cooperation.
Below are key points from the consultation on consistent policies and procedures as an approach to regulatory cooperation:
- Member economies acknowledge the value of consistent policies for cooperation and harmonization. They also see capacity and political issues as the challenges that will prevent more economies from exploring efforts to achieve consistent policies and assessments.
- International standards are seen as useful tools for overcoming these challenges and important for working towards alignment of policies and assessments for agbiotech. Most APEC member economies have at least a little experience with information and guidance from international forums and have at least taken these into consideration in their policy development.
- Codex Alimentarius is the most noted example, as is the work of the OECD Working Party on Safety of Novel Foods and Feeds and the OECD Working Party on Harmonization of Regulatory Oversight in Biotechnology. Member economies may be less familiar the information from the International Plant Protection Convention and the World Organization for Animal Health or information on risk/safety assessment found in Annex III of the Cartagena Protocol on Biosafety, and these may be less useful in efforts to align policies.
- Member economies see the value in alignment on food and feed safety, as well as environmental risk/safety assessments, and they acknowledge that this may be easier to accomplish for food and feed safety, largely because there are accepted international guidance for these from CODEX and from OECD.
- Economies recognize that there are some worthy examples of harmonization of approaches to food and feeds safety assessment between economies, including most notably the adoption of common guidelines in Mercosur and the process for joint food safety assessments developed by FSANZ and Health Canada, and see value in learning from these and other examples of policy alignment.
- To pursue policy alignment, APEC member economies might have more success by identifying a subset of economies, grouped according to some criteria, such as regional similarities or common language, or with similar systems in place for cultivation and food and feed, or at a similar stage of advancement of the technology.
- Member economies also acknowledged the importance of trust and taking into consideration existing relationships and past experience with policy alignment, other than biosafety policies, among economies.
III. C. Collaboration on Risk/Safety Assessments
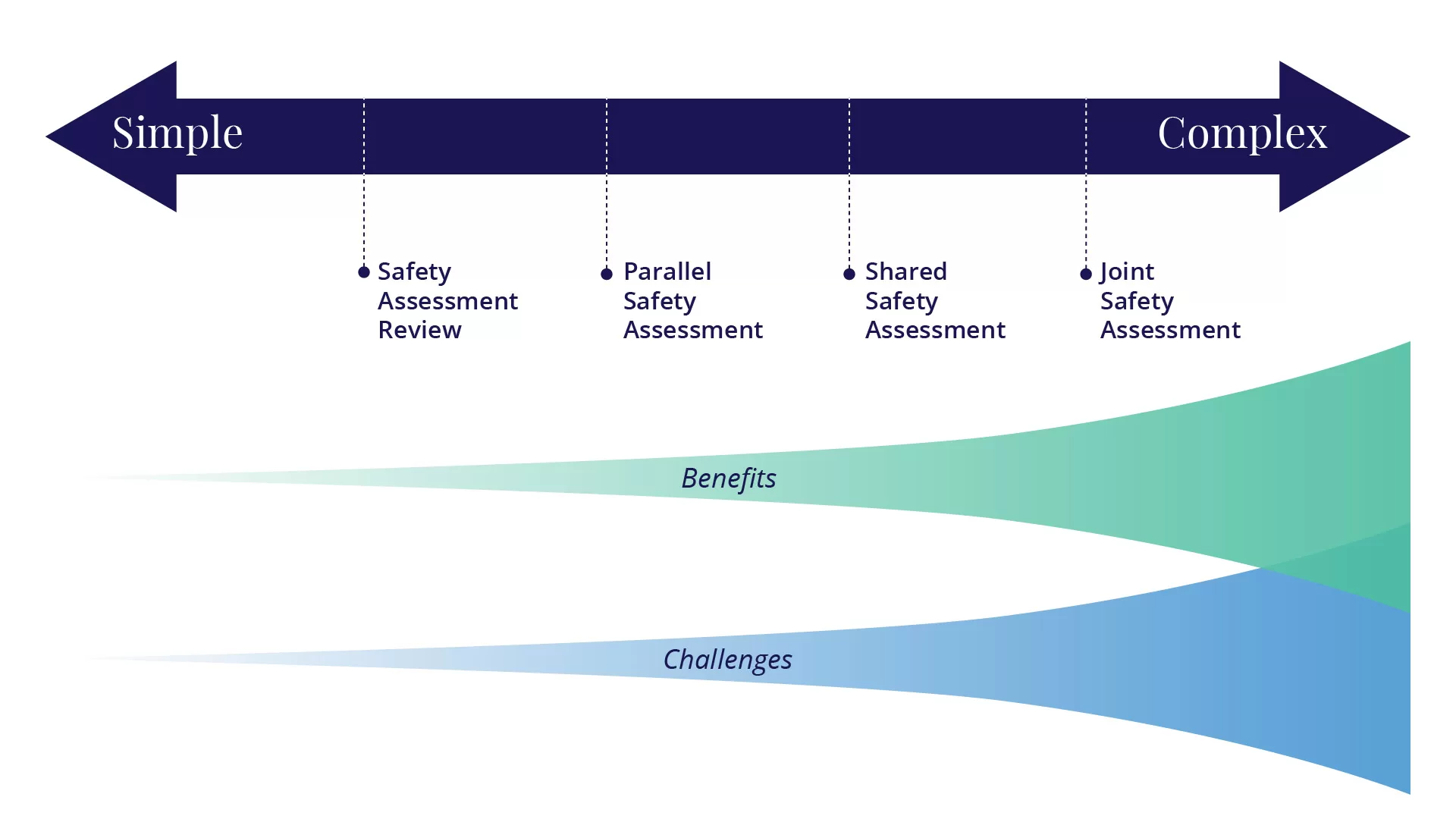
Possibly the most challenging approach to regulatory coordination for APEC economies to consider is collaboration on risk/safety assessments. There are a number of different ways to collaborate on risk/safety assessments, as described in this section: safety assessment review, parallel safety assessment, shared safety assessment, and joint safety assessment. These options and the benefits and challenges of each are also described in the OECD document on considerations for collaborative work on safety assessments of food and feeds derived from rDNA plants (OECD, 2021).
Benefits and Challenges of Collaboration on Risk/Safety Assessment
Some of the benefits and challenges that can be associated with collaboration on risk/safety assessment are described below. The benefits of these generally increase as the complexity at multiple levels also increase (Figure 1). As APEC member economies evaluate the feasibility of pursuing collaborations on risk assessment, they will want to consider how these different benefits and challenges factor into the process, depending on the type of collaboration being considered.
Below are some benefits of collaboration on risk/safety assessment:
- Efficiency gains in the assessment process
- Potential to reduce regulatory burden
- Improved synchronization of authorizations
- Mutual capacity building and learning
- Stronger working relationships among economies
- A regulatory environment that supports innovation
- Increased public confidence in regulatory decisions
Below are some challenges of collaboration of risk/safety assessment:
- Legal issues and legislative frameworks
- Operational differences between agencies
- Logistical and practical challenges
- Initial demand on resources
- Level of commitment
- Level of experience and expertise
Safety Assessment Review
Inter-agency peer review as a way to collaborate on risk/safety assessment is plainly a matter of agencies from different member economies having an arrangement in place to share safety assessments with each other for review. It is an example of sharing information through direct communication, as described earlier in this document. Although sharing safety assessments for review is also part of other types of risk/safety assessment collaboration, it can be in itself a form of collaboration. As part of the agreement, the reviewing agency may be expected to share comments or a critique of the assessment. Member economies may enter into such an arrangement in the interest of providing some assurance that the review and the review process are credible and acceptable according to pre-determined standards. Sharing safety assessments for review can also be a particularly useful tool for capacity building when more advanced member economies agree to share safety assessments with less experienced economies. In some cases, economies may choose to accept the review of another agency for their own decision-making, as described in the section on technical policy alignment.
Following a review of a safety assessment, an economy may also choose to consider the safety assessment, all or in part, for their own decision making of a given biotech product, as a simplified procedure to risk/safety assessment. In these cases, the economy accepting the review of another will have to coordinate closely with the assessing economy to understand the technical policies for safety assessment and ensure alignment of the process with their own. For this process to work, the reviewing economy will establish clear criteria, based on the expectation for an assessment, that must be met for the economy to accept the assessment for a decision. The review process then becomes a matter of reviewing the assessment against the established criteria.
Parallel Safety Assessment
This type of collaboration on risk/safety assessment is a pre-determined process by which two or more member economies complete their own safety assessment, but they do this in a coordinated fashion according to a mutually agreed timeline. It is similar to inter-agency peer review but requires considerably more commitment from the member economies involved because there would be a timeline in place and regularly scheduled inter-agency discussions to complete the review within a prescribed period of time. More time would be required by the member economies to compare and contrast their established approaches to risk/safety assessment before agreeing to this type of collaboration. This arrangement would also require cooperation of a product developer willing to submit an application simultaneously to both economies.
Shared Safety Assessment
Shared safety assessments are assessments developed together by two or more economies, where one economy takes the lead on drafting the risk/safety assessment and this draft is shared with another member economy for their review. The collaborating agencies then work jointly to finalize the assessment, and this final safety assessment is used independently by the agencies in their decision-making process. As with the parallel safety assessment, this type of collaboration requires a time investment up front to compare each economy’s approach and requires an agreement with the product developer.
Joint Safety Assessment
The joint safety assessment combines the elements of all previously described ways to collaborate on risk/safety assessments. In this type of collaboration, two or more economies conduct an assessment simultaneously, with each agency taking the lead on specific elements of the assessment. Once the draft is completed, it is reviewed and finalized by all economies involved and the final assessment is made independently by the agencies in their decision-making processes. This approach requires the most commitment from each of the collaborating economies, as all economies involved must contribute expertise and follow a close timeline in order to finalize the assessment.
Collaboration on Risk/Safety Assessment - APEC Opportunities and Challenges
A webinar consultation on sharing risk/safety assessments and procedures held on June 17, 2024 was attended by 12 participants from five member economies: Canada, Indonesia, Japan, Peru, and Philippines. A discussion with participants during this webinar provided some interesting insights regarding the value of and capacity for sharing risk/safety assessments and product approvals among member economies as a form of regulatory cooperation.
Below are the key points from the consultation on sharing risk/safety assessments and procedures as an approach to regulatory cooperation:
- Although it seems likely that APEC member economies would benefit from collaborative risk/safety assessment, most do not have experience working with other economies in this way. Member economies see the usefulness of collaborating to carry out risk/safety assessment of an agricultural product, but they do not see clearly how this can be accomplished. A few member economies have experience working with other economies toward collaborative risk/safety assessment of agricultural biotechnologies, but most have very little or none at all.
- Member economies see a clear benefit from pursuing some sort of collaboration on risk/safety assessment for reducing regulatory burden and increasing efficiency with regards to time but may not necessarily see the potential benefit of resource savings or reducing trade barriers.
- Although there is likely to be some resource savings for the regulatory community, mainly in terms of the cost of people’s time, it is worth considering what more opportunities might be presented to the product developers to save resources that might be associated with collaborative risk/safety assessments, and how that will benefit the economy.
- Some APEC member economies understand the potential benefit of synchronization of approvals and the benefit of this, particularly to facilitate movement of biotech products between economies, but most have not fully considered the important economic benefits associated with the reduction in trade barriers that is a likely benefit of many of the collaborations on risk/safety assessment.
- Regardless, member economies will need to weigh the benefits of pursuing such an approach against the challenges for establishing a working collaboration.
- Among the challenges member economies see, the greatest is reconciling differences between the economies. This includes differences in legislation and procedures, protection goals and other priorities, and differences in information and data requirements, as well as environmental and cultural differences that could factor into the risk/safety assessment process.
- The time and resources required to engage in collaboration on risk/safety assessment is also seen as a significant challenge.
- At a more nationalistic level, perceptions about maintaining sovereignty could present challenges. For collaborative risk/safety assessments to work, it will be important to clearly distinguish the shared process of risk/safety assessment from the independent process of decision-making to ensure sovereignty is retained.
- APEC member economies that choose to embark on a collaborative risk/safety assessment will need guidance on how to initiate a collaboration. The first steps include understanding the options for collaboration (as described in this document), and identifying key economies for collaboration by comparing systems, identifying similarities and differences, and determining how these might factor into efforts to collaborate on risk/safety assessment.
- Collaboration on risk/safety assessment is only possible if there is a shared commitment by the economies involved to pursue the collaboration. In addition, willingness of product developers to pursue collaborative risk/safety assessment should be taken into consideration.
- Another perceived benefit of cooperation on risk/safety assessment is the opportunity this presents for learning and building capacity. Economies with less experience could learn about risk/safety assessment from economies with more experience. While this is true, this is not a primary goal of collaboration on risk/safety assessment.
- Collaboration on risk/safety assessment is much more likely to be successful among economies that have a similar level of experience. There is an element of capacity building that comes with it, but it is not the purpose for collaboration on risk/safety assessment between member economies. Capacity building for risk/safety assessment is a form of regulatory cooperation worth pursuing among member economies as described in section III. A. on “information sharing.”
IV. Case Studies - Best Practices and Lessons Learned
The most up-to-date versions of available case studies can be accessed at: biotechpolicyportal.org/case-studies/
V. Future Direction
The APEC HLPDAB has recognized common interests and challenges among and between member economies related to implementing efficient and predictable trade in agricultural biotechnology with transparent and effective safety regulation. This document reflects the culmination of many years of discussion. The next steps will depend on the appetite and support within economies for moving towards practical implementation of regulatory cooperation in agricultural biotechnology.
As illustrated in Figure 1, a variety of options for collaboration may be considered, with the potential benefits as well as the difficulties increasing as the mechanisms for cooperation become more complex.
Information Sharing: The HLPDAB already serves as a forum for sharing information between and among APEC economies. The construction of the Policy Approaches Web Portal provides additional opportunities to formalize and enhance this information exchange, particularly through the provision of references and case examples of regulatory cooperation in the existing database, and those that might be added. Member economies may also wish to articulate additional ways and means that sharing information might be helpful to achieve the goals of the HLPDAB. This could include efforts to share the results of decision making processes or risk assessment outcomes. Consultations in preparation for the PAD also identified the opportunity to work on systemic challenges to information sharing. For example, developing mechanisms to address handling of confidential information to enable exchanges between APEC economies.
Policy Alignment: Member economies are already largely aligned on high level policy goals, although there are some exceptions. It is also clear that the priorities for implementation may be different amongst member economies. However, enough economies have sufficient high level policy alignment to enable future efforts for policy alignment at the technical and international level. Technical policy alignment includes efforts to collaborate around data requirements, including potential methods of data collection and analytical processes. This may be an especially robust opportunity for economies who have similar agricultural economies (e.g., primarily importing economies, primarily producing economies, etc.) and therefore, have commonalities in the kinds of agricultural biotechnology applications and types of reviews they need to conduct. Alignment around food safety assessment is strongly supported by the Codex Alimentarius, which provides technical guidance. This could facilitate collaboration around application formats or information collection in support of safety assessments.
Collaboration on Risk/Safety Assessment: Several examples of collaboration on risk/safety assessment have been included in the database on the Policy Approaches Web Portal, including examples being employed by APEC economies. Most notable is the safety assessment collaboration between FSANZ and Health Canada. This could serve as a model for bilateral or multilateral cooperation around food safety assessment among APEC economies with similar capacity and needs regarding food safety assessments. Again, facilitated by the Codex Alimentarius guidelines, there has been uniform outcomes for safety assessments of the products of biotechnology, suggesting that the results of future food safety assessments are likely to be the same regardless of the administrative procedure or the implementing agency.
APEC member economies are encouraged to consider creating an enabling environment by addressing barriers to regulatory cooperation, which have been identified. This could include exploring avenues for the exchange of information for the purpose of building understanding of risk/safety assessment processes in potential collaborating economies, raising awareness within member economy institutions about the potential benefits of risk/safety assessment collaboration, and providing a pathway to agreement from applicants to participate in assessment sharing procedures among economies. With a concerted effort, there are significant benefits to realize from regulatory cooperation among member economies.
Footnotes:
- Miami Winter Symposium. Nat Biotechnol 1, 36–38 (1983). https://doi.org/10.1038/nbt0383-36↩
- James, Clive. 2015. 20th Anniversary (1996 to 2015) of the Global Commercialization of Biotech Crops and Biotech Crop Highlights in 2015. ISAAA Brief No. 51. ISAAA: Ithaca, NY. ↩
- For example, the U.S. “Coordinated Framework for the Regulation of Biotechnology” was first published in June 1986.↩
- Baseline Review of APEC Member Economies’ Regulations of Products Derived from Innovative Agricultural Technologies (https://www.apec.org/docs/default-source/Publications/2019/6/Update-of-the-APEC-Baseline-Study/219_HLPDAB_Update-of-the-APEC-Baseline-Study.pdf) ↩
- https://www.wto.org/english/tratop_e/agric_e/ag_imp_exp_charts_e.htm ↩
